autoclave challenge pack|steris scbi challenge pack : China VERIFY Biological/Integrator Challenge Packs are used to monitor dynamic air removal (prevacuum and steam-flush pressure-pulse) steam sterilization cycles performed at . There are certain applications, such as sterilizing porous loads or partially vented containers, where air cannot be easily displaced from an autoclave chamber, rendering gravity air displacement ineffective. These loads — which may . See more
{plog:ftitle_list}
Wiley Online Library
a V-PRO Sterilizer in less than four hours. VERIFY ™ V24 Challenge Packs provide the confidence you need that your V-PRO Low Temperature Sterilization Systems are functioning .VERIFY Biological/Integrator Challenge Packs are used to monitor dynamic air removal (pre.The VERIFY ® All-In-One STEAM Reusable Test Pack, when used as a biological indicator .VERIFY Biological/Integrator Challenge Packs are used to monitor dynamic air removal (prevacuum and steam-flush pressure-pulse) steam sterilization cycles performed at .
A biological indicator process challenge device (BI test pack/PCD) should create a challenge to sterilant penetration that is less stringent than the load contents and placed in the least .3. After completion of the cycle, while wearing heat resistant gloves, retrieve the challenge pack. 4. Check to see that the external process indicator on the outside of the challenge pack has changed from yellow to brown or darker. Open the challenge pack and allow the 1492V BI to cool outside the challenge pack for 10 minutes prior to .
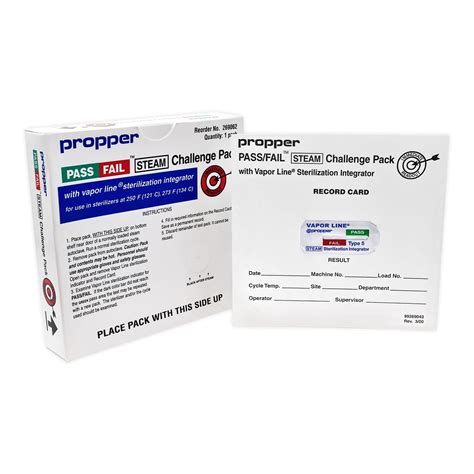
PCD26-C / Process Challenge Device with moving-front Type 5 Chemical Indicator for monitoring autoclaves. Integron® PCD26-C Process Challenge Device (PCD) Test Pack has been designed to simulate a load to be sterilized and to pose a challenge to the sterilization process. It is used to evaluate the effective performance of the process by detecting inadequate air removal and .The test pack is placed toward the bottom front of the loaded autoclave chamber near the drain. After the cycle has completed, technicians should immediately remove the challenge pack from the sterilizer and allow cooling before interpreting the chemical indicators and incubating the biological indicator for 24-hours before final results are read.
steris scbi challenge pack
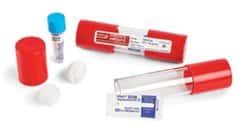
Process challenge devices (PCDs, also called test packs) are used to assess the performance of a sterilization process by providing a challenge to the process that is equal to or greater than the challenge posed by the most difficult item routinely processed.1 Effective performance of a steamThe 3M™ Attest™ Super Rapid Readout Steam Biological Indicator Challenge Pack with Type 5 CI 41482/4182V/4182VF, is a pre-assembled, white-wrapped test pack designed to monitor 270°F/132°C to 275°F/135°C dynamic-air-removal saturated steam sterilization cycles. Each pack includes a 3M™ Attest™ Steam Chemical Integrator, Type 5, and a 3M™ Attest™ Super . A process challenge device is a key element in the quality assurance testing of dental office sterilizers. It is used to monitor the performance of the sterilization process. The process challenge device simulates an equal or greater challenge than the most difficult instrument/item routinely processed in a sterilization cycle.
Study with Quizlet and memorize flashcards containing terms like The process by which all forms of microbial life are destroyed is, The sterilizer's heat-sensing thermometer is located in the:, In non-tabletop steam sterilizers, where are PCDs placed .
Load the autoclave. CHECK DRAIN SCREEN . a. Review the Standard Operating Procedures (SOP) for the autoclave unit. Training must be provided for any new autoclave operators. b. Check the drain screen at the bottom of the chamber before loading the autoclave. c. Place a piece of autoclave tape (Class I Chemical Indicator) on the outside of the3M™ Attest™ steam chemical integrators confirm that each pack in a load has been exposed to the conditions necessary for sterilization. Steam chemical integrators offer an immediate, accurate and easy to read way to monitor sterilization conditions inside each pack. . We can help you choose the right Bl Process Challenge Device (PCD .Where should the biological test pack be placed on the steam sterilization cart for the first run of the day? A. Top rack, back of sterilizer, under the injection valve. B. Bottom rack, back of sterilizer, under the injection valve. C. Top Rack, front of sterilizer, over the drain D. Bottom rack, front of sterilizer, over the drainFor larger autoclaves, the spore test is performed using a commercially-available challenge test pack, also known as a process challenge device. The purpose of this is to mimic the situation for instruments/devices being sterilized. It consists of a test pack or tray containing the biologic indicator. What is sterility assurance?
Product Overview. The Celerity 20 STEAM Biological Indicator (BI) is a fast-read 20-minute BI used for routine monitoring, qualification testing, load monitoring and product testing of commonly used steam sterilization cycles.. How the Celerity 20 STEAM Biological Indicator Works. After a steam sterilization cycle, the cap's color-changing process indicator will provide immediate .Order Verify® Sterilization Indicator Challenge Pack Steam 4 Inch Length by Steris LCB007. Pharmaceuticals Laboratory Government McKesson Medical Supplies . Challenge Packs are used to release dynamic air removal (prevacuum and steam-flush pressure-pulse) steam sterilization (autoclave) cycles performed at 270°F/132°C and gravity cycles .The challenge pack is FDA-cleared, and the biological indicator (BI) is ISO-compliant and must be used with the 3M™ Attest™ Auto-reader 490 or 490M. . AQUASTAT produces the pure water necessary for autoclave sterilization in your office; The fast distilling and energy efficient AQUASTAT processes 3.78 litres (1 gallon) of distilled water .
Process Challenge Device with self-adhesive Type 5 Chemical Indicator for monitoring autoclaves. Process Challenge Device Integron® PCD26-2 Test Pack was developed to detect inadequate air removal and steam penetration in dynamic-air removal (pre-vacuum) steam sterilizers at 132/135 °C 4 minutes and gravity displacement steam sterilizers at 121 °C 30 . Commercially available disposable test packs that have been shown to be equivalent to the AAMI 16 towel test pack also may be used. The test pack should be placed flat in an otherwise fully loaded sterilizer chamber, in the area least favorable to sterilization (i.e., the area representing the greatest challenge to the biological indicator).Celerity™ HP Challenge Packs Read Time: Provides biological indicator results in as little as 5 minutes; Cycles: Used for qualification of V-PRO™ Low Temperature Sterilizers in about 3 hours; Feature: Challenge pack includes a Celerity™ HP Biological Indicator, a chemical indicator, and a blue polyurethane foam sheet
A sterilization pouch, or peel pack, is a disposable package used in a sterilizer to allow penetration of the sterilant to the items placed inside. After sterilization, these Class II Medical Devices maintain the sterility of the processed item. . Examples include several small items or instruments that might present a challenge during .
Celerity™ HP Challenge Packs. Read Time: Provides biological indicator results in as little as 5 minutes Cycles: Used for qualification of V-PRO™ Low Temperature Sterilizers in less than three hours Feature: Challenge pack includes a Celerity™ HP Biological Indicator, a chemical indicator strip, and a blue polyurethane foam sheet
Order Verify® Sterilization Indicator Challenge Pack Steam 4 Inch Length by Steris LCB006. Pharmaceuticals Laboratory Government McKesson Medical Supplies . Challenge Packs are used to release dynamic air removal (prevacuum and steam-flush pressure-pulse) steam sterilization (autoclave) cycles performed at 270°F/132°C and gravity cycles .“PCDs are challenge test packs containing a BI or BI and a CI. A PCD is used to assess the effective performance of a sterilization process by providing a challenge to the process that is equal to or greater than the challenge posed by the most difficult item routinely processed” (p. 53).PCD26-C / Process Challenge Device with moving-front Type 5 Chemical Indicator for monitoring autoclaves. Integron® PCD26-C Process Challenge Device (PCD) Test Pack has been designed to simulate a load to be sterilized and to pose a challenge to the sterilization process. It is used to evaluate the effective performance of the process by detecting inadequate air removal and .
They are used in PCDs (i.e., test packs or challenge packs) for the purpose of Load Control. The BI PCD is placed in the most challenging location in the sterilizer with a full load and provides information about the efficacy of the sterilization cycle. Routine sterilizer efficacy testing with a BI PCD is recommended weekly, preferably daily, andThe Process Challenge Device(PCD) packs are used to monitor steam sterilization loads and can be used to release non implant loads. The PCDs are designed for use in standard steam sterilizing processes, including 250° F (121°C) gravity steam, 270°F (132°) prevacuum Each pack consists of a series of steam penetration and air removal barriers.
sterilisation challenge pack
STERIS Verify Process Challenge Devices (PCD) are pre-assembled challenge test packs that combine a Self Contained Biological Indicator (SCBI) and a chemical indicator inside a complicated outer package. The outer package provides a torturous path that is difficult to sterilize and presents a significant challenge to your sterilization process.
Sterilizers and Autoclaves Sterilization Verification Indicators Steris™ VERIFYtrade; Biological Steam Test Pack (SCBI) with Control . (SCBI) with Control. Steris Corp™ VERIFY™ Biological/Integrator Challenge Pack used to monitor and qualify autoclave cycles .00 - .30 Specifications. Amperage: 0.5 A: Dimensions (L x W) 82.55 x 29 mm:Place packs in the direction of steam flow, don't fully pack the autoclave, and allow contents to dry for at least 20 minutes. 1 / 29. 1 / 29. Flashcards; Learn; Test; . Ch. 15 & 16 challenge Qs. 75 terms. rodriguez7129. Preview. Diagnostic Imaging. 33 terms. catesandvik1. Preview. Intro to radiology. 13 terms. fionat4ng. Preview .
alcon schirmer tear test strips
$869.99
autoclave challenge pack|steris scbi challenge pack